今天想给介绍一些CCC竞赛真题,这份是来自2年前的真题,同学们在备考的时候一定要紧抓真题,根据真题的题型和知识点来复习。
CCC竞赛真题
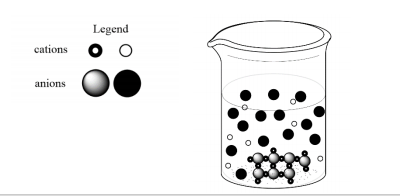
A student combines 100 mL of two clear, equimolar, colourless solutions and observes a solid white precipitate form at the bottom of the beaker. A
representation of the resultant particles in the beaker is in the diagram below. Which combination of reactants would best align with the information
provided and diagram of resultant particles in the beaker?
A) lead (II) nitrate (aq) + potassium chloride (aq)
B) zinc nitrate (aq) + sodium phosphate (aq)
C) nickel (II) nitrate (aq) + lithium bromide (aq)
D) copper (II) nitrate (aq) + sodium hydroxide (aq)
E) silver nitrate (aq) + magnesium sulfate (aq)
A student adds two moles of liquid water initially at 273 K to three moles of liquid water at 363 K in a perfectly insulated container. The total volume
of water remains constant. Assume that the molar heat capacity of liquid water is constant and independent of temperature. What is the final
equilibrium temperature of the water?
A) 298 K B) 309 K C) 318 K D) 327 K E) 358 K
One mole of uranium-238 decays slowly. If uranium-238 decays according to first order kinetics, after how many half-lives is there likely only one
atom of uranium-238 left?
A) 85 B) 10 C) 238 D) 41 E) 79
Which of the following should have the same electron arrangement as BF4-around the central atom?
A) IF4– B) XeCl4 C) ClF4+ D) SF4 E) CCl4
A mixture of ethanol and nitric acid, called nital, is an industrial etching agent. A student prepared a 20.0 mL solution of nital using 0.70 mL of nitric
acid of unknown concentration and 19.3 mL of 98% ethanol. The student determined by titration that the final concentration of nitric acid in the nital
etching agent was 4.0 % by mass. The density of 98% ethanol was 0.79 g mL-1 and the density of the unknown concentration of aqueous HNO3 was
1.4 g mL-1. What was the original concentration of HNO3, in w/w, in the0.70 mL reagent the student used for the nital solution preparation?
A) 63% B) 66% C) 70% D) 73% E) 93%
CCC竞赛真题就分享到这里。既然是拔高类竞赛,难度肯定不小,不仅需要具备A-level、IB或者等同的化学知识储备,而且考试中很多部分的知识点学员上课未涉及过的,是校内化学课程的延伸。如果你打算参加CCC等国际竞赛,但不知道如何针对性复习,可以点击预约试听【唯寻竞赛复习班】——

点击
加拿大化学竞赛CCC考试内容有哪些?这8个模块的内容可要记牢了
查看。












































 沪公网安备 31010502004453号
沪公网安备 31010502004453号





 成功提交后我们将尽快与您联系,请注意来电!
成功提交后我们将尽快与您联系,请注意来电!






 成功提交后我们将尽快与您联系,请注意来电!
成功提交后我们将尽快与您联系,请注意来电!


