
复习AP化学的小伙伴,都少不了AP化学FR真题的助攻。本文就来为大家分享一下2018年的4道AP化学FR真题。即将到来的holidays是今年AP考生可以大段利用的时间,所以一定要认认真真刷完真题这样的宝贵复习资源呀。
AP化学考试自由作答题(FRQ)的前面三题综合性比较强,分值较高;后面四题相对简单,全部可以使用计算器。AP化学对于计算器要求不高,一般 科学计算器就够用了;图形计算器也可以使用。
根据CollegeBoard官方解析,化学简答题的题型通常包括如下类型:
1.Experimentaldesign实验设计
2. Analysis of authentic lab data and observations to identify patterns or explain phenomena分析真实的实验室数据并观察结果,以识别某种模式或解释某种现象
3. Analysis of authentic lab data and observations to identify patterns or explain phenomena 创建或分析原子和分子视图以解释观察结果 ‘
4. Articulating and then translating between representations 清晰表达并翻译不同的展示图像5. Following a logical/analytical pathway to solve a problem 使用逻辑/分析途径来解决问题
题
解析:
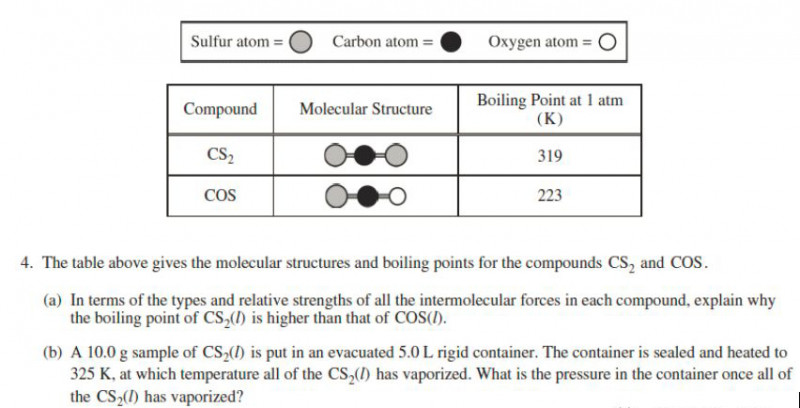
(a) For nonpolar CS2 molecule, there is London dispersion force; for polar COS molecule, there are London dispersion force and dipole-dipole force. Since CS2 has a larger molecular mass and more electrons than COS, CS2 has stronger London dispersion force than the combination of London dispersion force and dipole-dipole force in COS. Therefore, CS2 has a higher boiling point than COS.
(b) PV = nRT
P = nRT / V = 10.0g x (1mol/76.13g) x 0.08206 L atm mol-1 K-1 x 325K / 5.0L = 0.70 atm
第二题
解析:
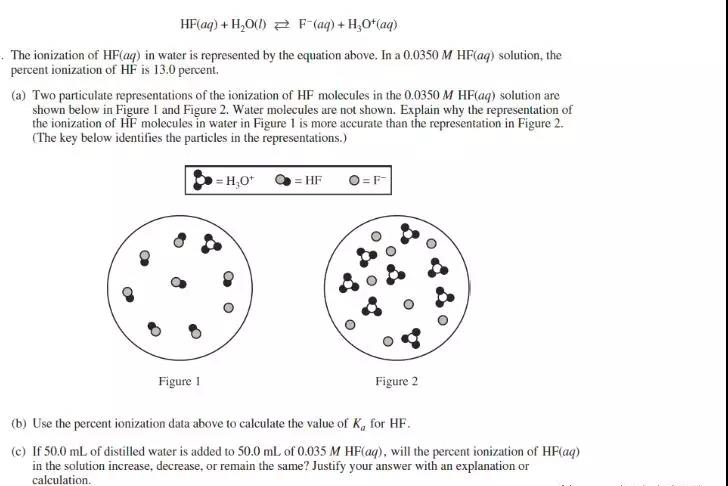
(a) Since percent ionization of HF is 13.0%, most particles containing F should be in the form of unionized form HF at equilibrium, which means that HF is a weak acid and only partially ionized in water. Figure 1 shows partial ionization of HF. In figure 2, all HF molecules are completely ionized into H3O+ and F-, which corresponds to a strong acid.
(b)

Ka = [F-][H3O+]/[HF] = 0.004552/0.03045 = 6.8 x 10-4
(c) The percent ionization of HF in the solution will increase. When the solution is diluted, the concentration of the “particles” in the solution is reduced. According to Le Chatelier’s principle, this reduction in particle concentration is counteracted by shifting the reaction to the side with more particles.The equilibrium shifts from the nonionized acid side to the side containing the H+ ion and the conjugate base. The percent ionization of the acid increases.
第三题
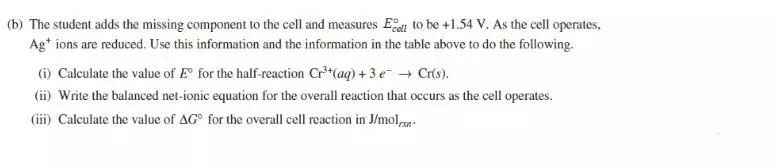
解析:
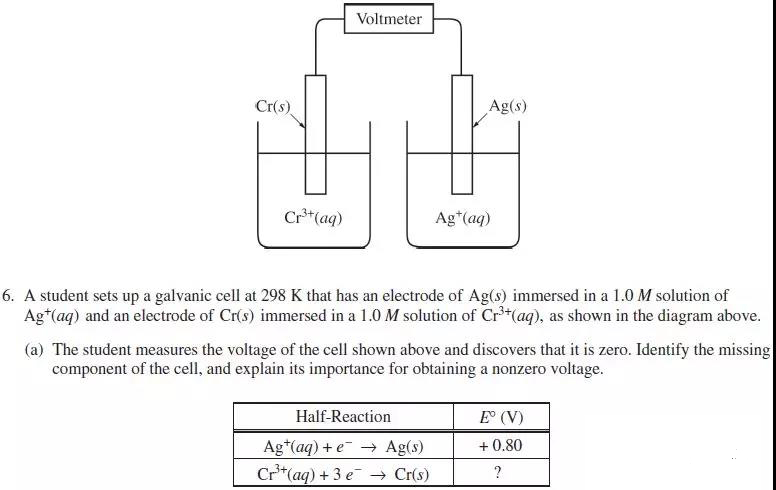
(a) The missing component of the cell is salt bridge. It allows electrical contact between the two solutions and the cell can form a closed circuit.
(b) (i) = = 0.80V - E0(Cr3+/Cr) = 1.54V
E0(Cr3+/Cr) = 0.80V – 1.54V = -0.74V
(ii) 3Ag+(aq) + Cr(s) -----> 3Ag(s) + Cr3+(aq)
(iii) ∆G0 = -nFE0 = - 3mol x 96485 C/mol x 1.54V = -4.46 x 105 J
第四题
The complete photoelectron spectrum of an element is represented above. (a) Identify the element.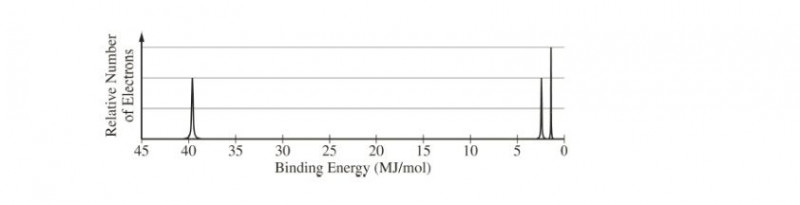
A radioactive isotope of the element decays with a half-life of 10. minutes.
(b) Calculate the value of the rate constant, k, for the radioactive decay. Include units with your answer.
(c) If 64 atoms of the radioactive isotope are originally present in a sample, what is the expected amount of time that will pass until only one atom of the isotope remains? Show how you arrived at your answer,
解析:
(a) N (7 electrons)
(b) t1/2 = ln2 / k
k = ln2 / t1/2 = ln2 / 10.min = 0.069 min-1
(c) 1/64 = (1/2)6, 6 x t1/2 = 6 x 10.min = 60.min
2018年AP化学FR真题就解析到这里,希望能帮助你熟悉AP化学的出题风格与考试节奏。FR对AP考试的意义相信大家都心知肚明,如果你刷完了上面的题目,觉得自己的FR做起来还不太顺,一定要早提问早解决,堆到4月份再来复习、培养解题思路肯定是来不及的。如果你觉得自己需要AP化学或者其他科目的专门辅导,欢迎点击预约试听【AP复习冲刺班】,专为2021年5月考试设计课程,唯寻教学天团授课,根据学员进度定制课程,从近年真题中解码考点,梳理高频知识点,提升答题技巧,同时复习多门科目,也能助力理想分N连。

不管是英国前10还是美国前20的学校,都非常认可AP成绩。但AP考试采取五分制,排名靠前的学校要求4-理想分才能换学分,也只有接近满分的成绩才能真的帮到申请。所以AP考生一定要以理想分为理想目标呀。
更多AP复习攻略点击

学习有方法,成长看得见
筑梦牛剑/G5/常春藤
