每年很多想申请国外化学、医学、药学、材料等专业的小伙伴,都会选择参加UKChO竞赛,来丰富自己的背景提升履历。距离2022年的比赛还有1个月的时间,本文就来拎拎复习的重点,祝大家一月的比赛都能赛出佳绩。
2022UKCHO竞赛时间
报名截止:2022年1月12日
考试时间:2022年1月22日(周六)下午14:00-16:00(120分钟)
题型为约5道分析简答答题,每道大题有3-10个小题,难度较大,所有试卷都会寄回英国评分,有RSC(英国皇家化学协会)指定考官进行评判。
简答题的题干还是很长的,大家可以看下2去年的真题感受↓
1.This question is about life on Venus In 2020, a controversial research paper announced the detection of phosphine (PH3) in the atmosphere of Venus. On Earth, phosphine typically comes from either biological processes or industrial processes and other than this is not produced ‘naturally’. Its possible presence on Venus indicates a potential biological origin, although the conditions on Venus are very different to Earth, with temperatures up to 698 K, pressures up to 95 atm and clouds of sulfuric acid.

(a)
Draw the shape of phosphine. Phosphine does not accumulate in Earth’s atmosphere as it reacts with oxygen to produce phosphoric acid (H3PO4). The atmosphere of Venus does not contain oxygen.
b)
(i) State the oxidation states of phosphorus in phosphine and phosphoric acid.
(ii) Write an equation for the reaction of phosphine with oxygen to make phosphoric acid. There are several methods to produce phosphine that do not require living organisms. These processes do not occur naturally on the Earth’s surface because the starting materials are not found or the conditions are not suitable. Write equations for the following reactions which all produce phosphine.
————————————————UKCHO真题分割线————————————————
(c)
(i) The reduction of phosphorus trichloride with lithium hydride.
(ii) The hydrolysis of calcium phosphide.
(iii) The high temperature disproportionation of phosphorous acid (H3PO3).
Another simple molecule often detected in space is methanal. Methanal is highly reactive, so any produced on Venus would be quickly consumed. Phosphine reacts with methanal under acidic conditions. In the presence of sulfuric acid, phosphine and methanal react to give a salt of molar mass 406.272 g mol−1 . The cation of this salt has an overall charge of +1. The 13C NMR of this salt has only one signal. The 1H NMR of this salt shows two signals, one of which exchanges with D2O.
(d)
(i) Write the formula of the anion.
(ii) Draw the structure of the cation.
如何准备UKCHO竞赛?
01 不要轻言放弃
UKChO没有考试大纲,但是学习过A-level化学的同学可以尝试一下。问题都比较有难度,需要运用所学知识,解决问题,做出合理猜测。不要轻易放弃,即使一个小分题,说不定就能助你过线
02 拓展一些有机知识
UKCHO已经不能算入门竞赛了,总体难度较高,考试内容和AS/A2化学有一定重合,同时对于有机反应部分有更多的拓展,对于AS阶段学生需要额外学习较多的有机部分内容。如果不是很清楚具体要多学哪些内容,怎样准备复习,可以点击预约试听【唯寻UKCHO竞赛复习班】——
根据学员进度定制课程
介绍联想、类比、归纳、转化、试误等答题技巧
整合大量竞赛全真题库
进行模块化学习
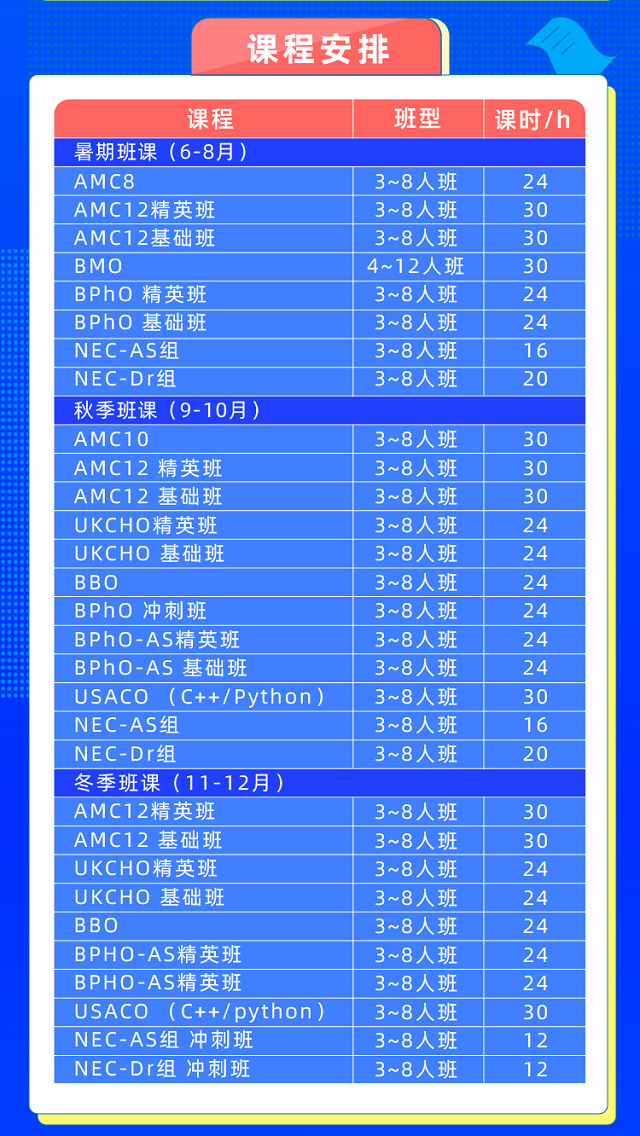

更多国际竞赛攻略点击↓











































 沪公网安备 31010502004453号
沪公网安备 31010502004453号





 成功提交后我们将尽快与您联系,请注意来电!
成功提交后我们将尽快与您联系,请注意来电!






 成功提交后我们将尽快与您联系,请注意来电!
成功提交后我们将尽快与您联系,请注意来电!


