
化学反应速率在IGCSE化学学习中出现在topic7,化学反应速率与我们平时的生活还是有些关系的,同学们想要探究平时生活中的化学现象的话,这些IGCSE化学反应速率知识点就一定要了解。
Rate of Reaction 的主要考点
1.Describe and explain the effect of concentration, particle size, catalysts (including enzymes) and temperature on the rate of reactions
2. (Extended only) Devise and evaluate a suitable method for investigating the effect of a given variable on the rate of a reaction
3. Describe the application of the above factors to the danger of explosive combustion with fine powders (e.g. flour mills) and gases (e.g. methane in mines)
4. Demonstrate knowledge and understanding of a practical method for investigating the rate of a reaction involving gas evolution
5. (Extended only) Describe and explain the effects of temperature and concentration in terms of collisions between reacting particles.
6. Interpret data obtained from experiments concerned with rate of reaction (use the term rate over speed)
7. (Extended only) Describe and explain the role of light in photochemical reactions and the effect of light on the rate of these reactions
8. (Extended only) Describe the use of silver salts in photography as a process of reduction of silver ions to silver; and photosynthesis as the reaction between carbon dioxide and water in the presence of chlorophyll and sunlight (energy) to produce glucose and oxygen
以上这些知识点简言之就是:
1.大块是温度,反应物浓度,反应物表面积和催化剂对化学反应速率的影响;
2.二是如何通过常见实验测量并比较化学反应速率;
3.三是通过碰撞理论解释几大影响化学反应速率的因素;
4.四是光化学反应的应用,例如银金属生成的盐如何应用在照相业以及光合作用的过程。
讲完了知识点,下面给大家带来了一些真题。
温度,反应物浓度,反应物表面积和催化剂对化学反应速率的影响
An experiment, S, is carried out to measure the volume of hydrogen produced when excess dilute sulfuric acid is added to zinc.
A second experiment, T, is carried out using the same mass of zinc but under different conditions. The results of the two experiments are shown.
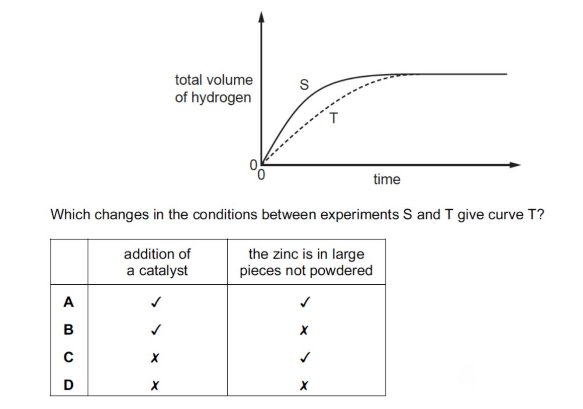
解析:这一题考查了催化剂和反应物表面积对反应速率的影响。曲线S的斜率比曲线T的斜率要缓,说明S的反应速率比较慢,但是最终两条曲线汇在了一起,说明这个加入的条件不会影响反应物最终的产量。那么在酸过量的前提下加入催化剂,或者改变反应物的表面积都不会改变最终生成的产物的量。Addition of catalyst 可以加快反应速率,但是 zinc in large pieces 是减小反应物面积,会减缓速率,所以本题T曲线是增快的了反应速率,那么应选B.
The results of two separate reactions between excess calcium carbonate and hydrochloric acid are shown.
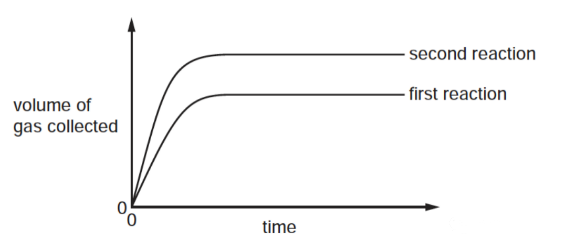
Which statement explains the differences between the reactions?
A More calcium carbonate was used in the second reaction.
B The same volume of more concentrated acid was used in the second reaction.
C The second reaction was allowed to react for longer.
D The temperature was higher in the second reaction.
解析: 这一题非常容易选错。我们首先来分析两条曲线,second reaction 比 first reaction 反应得到的气体要多,那肯定是反应物的浓度增加了,因为其他的例如温度,催化剂,反应物面积都不会改变最终的反应物生成量。那么我们可以直接排除C 和 D;A 和B中我们需要回到题目中,过量的是 calcium carbonate而不是hydrochloric acid,那么改变calcium carbonate的量是无法改变反应速率的,hydrochloric acid作为limiting reagent 才是改变反应速率和生成物浓度的关键,所以排除A,得到B选项。
When aqueous sodium thiosulfate and dilute hydrochloric acid are mixed, a precipitate of insoluble sulfur is produced. This makes the mixture difficult to see through.
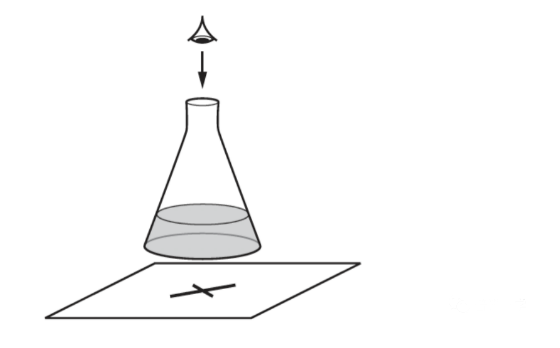
A student adds the following volumes of aqueous sodium thiosulfate, dilute hydrochloric acid and distilled water to the conical flask.
(a) State the order in which the aqueous sodium thiosulfate, hydrochloric acid and distilled water should be added to the flask.................................[1]
(b) In experiment 3 the student wanted the sodium thiosulfate to be double the concentration used in experiment 2.
(i) Complete the table to show the volumes which should be used, and the expected time taken for the cross to disappear from view in experiment 3. [2]
(ii) Use collision theory to explain why increasing the concentration of sodium thiosulfate would change the rate of reaction............................................ [2]
(c) The student repeated experiment 1 at a higher temperature.
Use collision theory to explain why the rate of reaction would increase......................................[3]
[Total: 8]
解析:
(a) 这一题问的是反应物的加料顺序,这一题只需要记住会反应的物质我们需要把他们隔开,否则无法记录反应速率,所以把distilled water放在中间,另外两个随意组合即可,如:sodium thiosulfate, distilled water, hydrochloric acid;
(b) (i) 题目中说sodium thiosulfate浓度double,那么体积就是原来两倍,从20 mL 变成40 mL,控制变量hydrochloric acid体积不变10 mL,那么为了保持总体的体积不变为60 mL,distilled water 的体积应该为10 mL;
(ii) 题目让我们用碰撞理论解释为什么升高浓度会加速反应,那么只需要记住下面的语句:
得分点1: more particles per unit volume / particles are closer together;
得分点2:increase the rate of reaction collisions
(c ) 题目让我们继续用碰撞理论解释为什么升高温度会加速反应,那么只需要记住下面的语句:
得分点1:particles gain more energy and move faster
得分点2: increasing rate of collisions
得分点3: higher proportion of particles have sufficient energy to react
IGCSE化学知识点就整理到这里。 关于今年大考有不少科目考纲有变化,想锁定A*还是很有难度的。如果你还不知道如何根据新考试变动调整学习计划,点击预约试听【唯寻IGCSE同步培训班】——
全球唯寻教学天团授课
紧贴不同考试局大纲变动
不断革新教学方法
研发教学产品及模块化练习资料
弥补以往学习过程中的遗漏点
提供行之有效的复习方案
点击
查看。

学习有方法,成长看得见
筑梦牛剑/G5/常春藤
